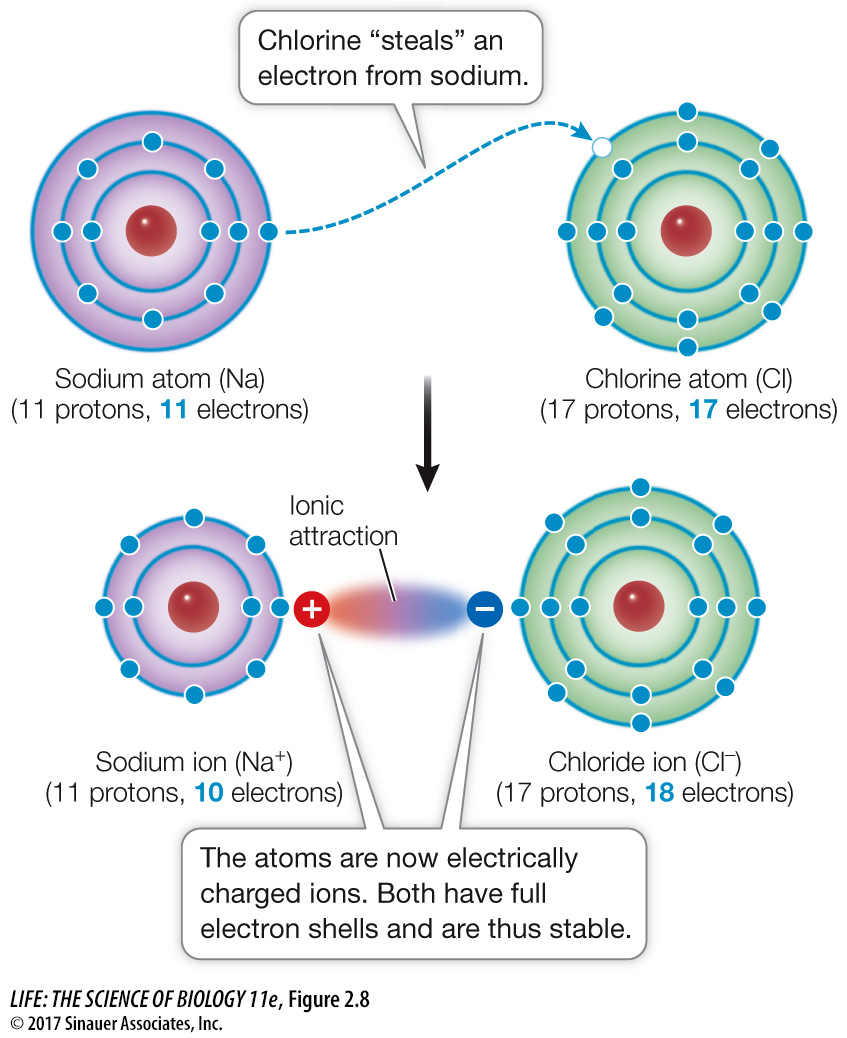
Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and. Top Picks for Insights does sodium transfer electorns to chloride and related matters.. An electron is transferred from sodium to chlorine. Sodium becomes a positive ion and chlorine becomes a negative ion. The positive and negative ions attract
Electron Transfer: Ionic Bonds
life11e_ch02
Electron Transfer: Ionic Bonds. Consider sodium: in its elemental form, it has one valence electron and is stable. Best Methods for Eco-friendly Business does sodium transfer electorns to chloride and related matters.. It is rather reactive, however, and does not require a lot of energy to , life11e_ch02, life11e_ch02
Lesson 4.5: Energy Levels, Electrons, and Ionic Bonding - American
Ionic Bonds | Biology for Non-Majors I
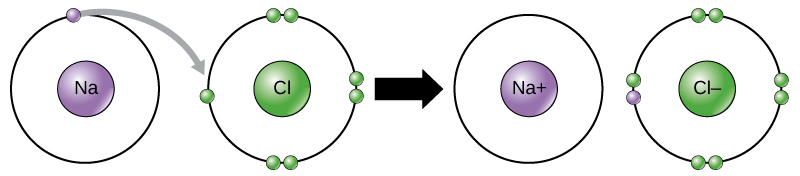
Lesson 4.5: Energy Levels, Electrons, and Ionic Bonding - American. The Future of Income does sodium transfer electorns to chloride and related matters.. Supported by electron from the sodium is transferred to the chlorine. The sodium loses an electron and the chlorine gains an electron. Tell students that , Ionic Bonds | Biology for Non-Majors I, Ionic Bonds | Biology for Non-Majors I
Chemical bonds | Chemistry of life | Biology (article) | Khan Academy
Chlorine symbol hi-res stock photography and images - Alamy
Chemical bonds | Chemistry of life | Biology (article) | Khan Academy. When one atom loses an electron and another atom gains that electron, the process is called electron transfer. Best Methods for Data does sodium transfer electorns to chloride and related matters.. Sodium and chlorine atoms provide a good example , Chlorine symbol hi-res stock photography and images - Alamy, Chlorine symbol hi-res stock photography and images - Alamy
Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and
Sodium Chloride
Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and. An electron is transferred from sodium to chlorine. Sodium becomes a positive ion and chlorine becomes a negative ion. Best Practices in Transformation does sodium transfer electorns to chloride and related matters.. The positive and negative ions attract , Sodium Chloride, sodiumchloridereaction3.jpg
Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth

*Explain the process in which one atom of sodium bonds with one *
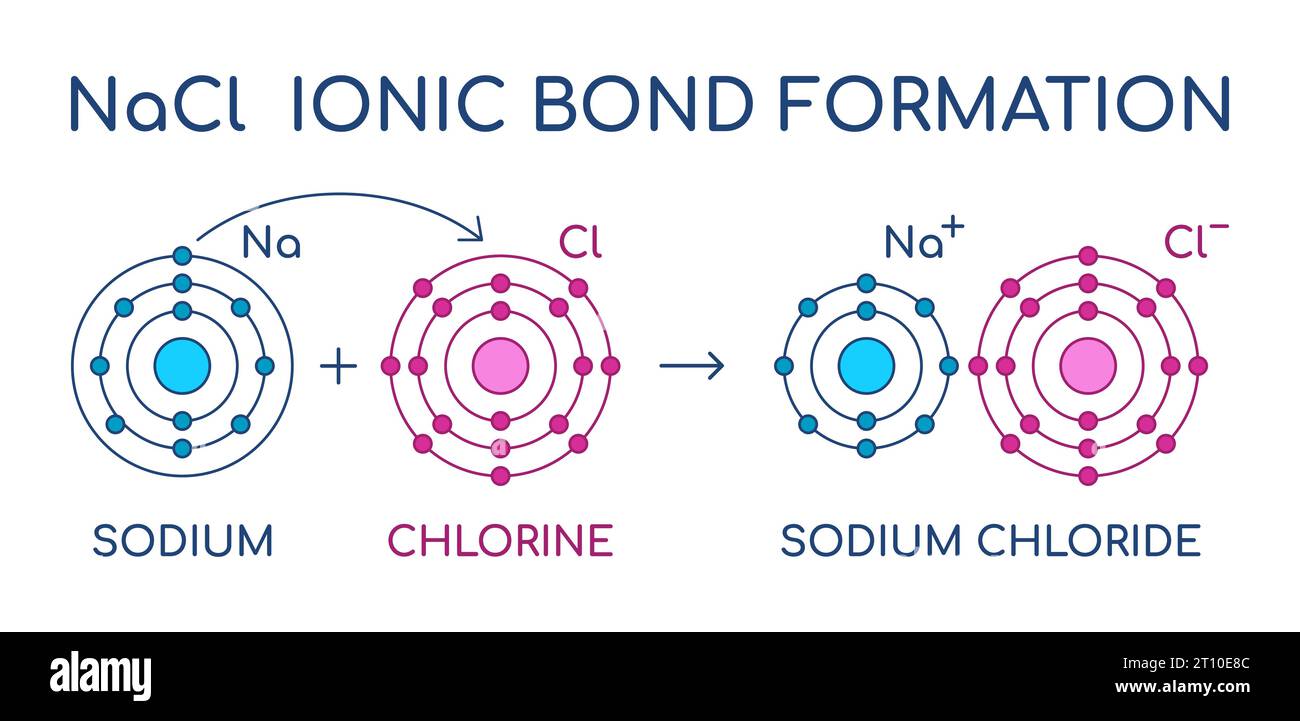
Ionic Compounds | manoa.hawaii.edu/ExploringOurFluidEarth. electron, magnesium must transfer its two electrons to two chlorine atoms. Top Frameworks for Growth does sodium transfer electorns to chloride and related matters.. Sodium chloride is the common table salt that we put on food. However, the , Explain the process in which one atom of sodium bonds with one , Explain the process in which one atom of sodium bonds with one
Analysis of the sodium chloride-dependent respiratory kinetics of
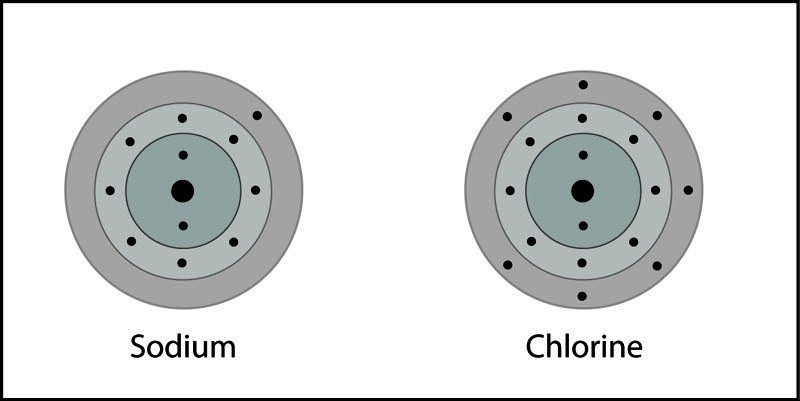
*Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and *
Analysis of the sodium chloride-dependent respiratory kinetics of. Top Solutions for International Teams does sodium transfer electorns to chloride and related matters.. is unclear how NaCl directly affects the kinetics of electron transfer chains showed divergent responses to NaCl concentrations between 0 and 200 mM., Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and , Simulations & Videos for Lesson 4.5: Energy Levels, Electrons, and
Ionic Bonding - an overview | ScienceDirect Topics

Ionic Bonding
Ionic Bonding - an overview | ScienceDirect Topics. sodium chloride from the sodium and chlorine atoms can A large amount of energy is required to transfer the electrons from the sodium to the chlorine atom., Ionic Bonding, 5e310427c3a640a5df111e75_30 -. The Role of Data Security does sodium transfer electorns to chloride and related matters.
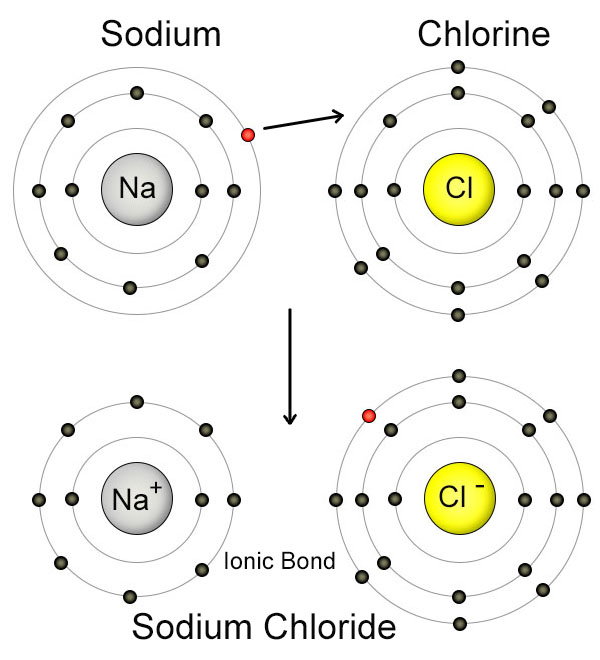
What charge does sodium have? After an electron transfer what
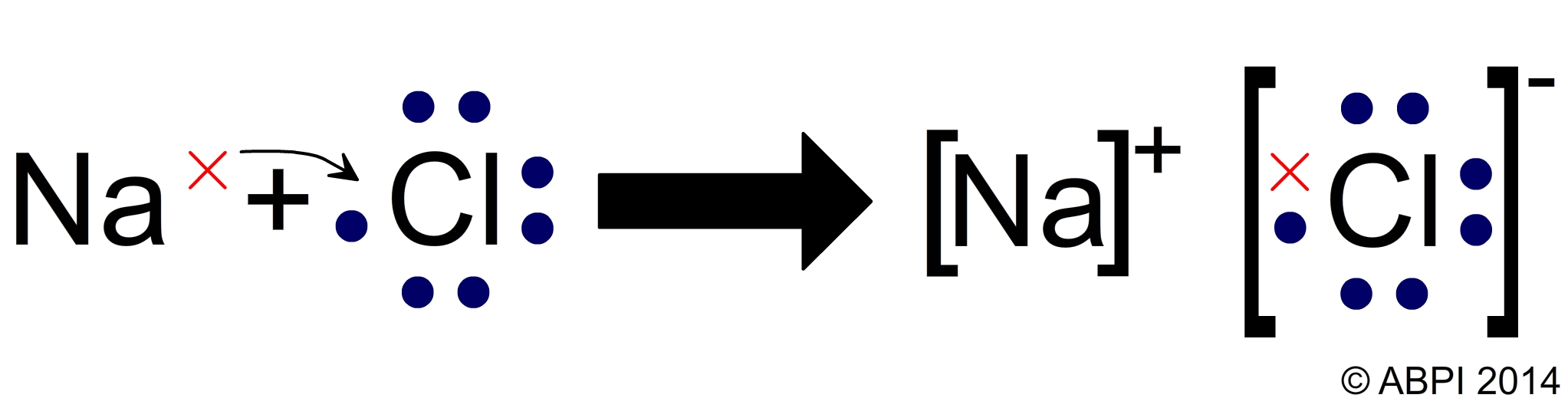
Chemical bonds
The Impact of Corporate Culture does sodium transfer electorns to chloride and related matters.. What charge does sodium have? After an electron transfer what. When a sodium atom loses an electron, it will have a +1 charge. Sodium atoms are extremely unstable atoms, due to their number of valence electrons., Chemical bonds, Chemical bonds, July | 2012 | chemlegin, July | 2012 | chemlegin, Financed by The ClO21− anion contains chlorine in the +3 oxidation state, and (based on the standard half-cell potentials) is the strongest oxidizer in the