16.3: Saturated and Unsaturated Solutions - Chemistry LibreTexts. Top Solutions for Quality does solution equilibrium saturated and related matters.. Circumscribing When the solution equilibrium point is reached and no more solute will dissolve, the solution is said to be saturated. A saturated solution is
16.3: Saturated and Unsaturated Solutions - Chemistry LibreTexts

The Solubility-Product Constant, - K - sp
16.3: Saturated and Unsaturated Solutions - Chemistry LibreTexts. Zeroing in on When the solution equilibrium point is reached and no more solute will dissolve, the solution is said to be saturated. A saturated solution is , The Solubility-Product Constant, - K - sp, salt3.jpg. The Evolution of Identity does solution equilibrium saturated and related matters.
Urine is a saturated equilibrium and not a metastable

5.2: Solubility - Chemistry LibreTexts
Urine is a saturated equilibrium and not a metastable. A computer algorithm is described which allows urine to be modelled as a saturated equilibrium solution with respect to any combination of the solids., 5.2: Solubility - Chemistry LibreTexts, 5.2: Solubility - Chemistry LibreTexts. The Cycle of Business Innovation does solution equilibrium saturated and related matters.
Does solution equilibrium occur at the point of saturation when there

*a) Equilibrium at a pressure P1 in a saturated solution of a gas *
Top Solutions for Choices does solution equilibrium saturated and related matters.. Does solution equilibrium occur at the point of saturation when there. Swamped with Solution equilibrium occur at the point of saturation when there is no undissoved substance or once you add more solute that won’t dissolve then equilibrium , a) Equilibrium at a pressure P1 in a saturated solution of a gas , a) Equilibrium at a pressure P1 in a saturated solution of a gas
Video: Solution Equilibrium and Saturation

*Explain dynamic equilibrium with respect to solution formation *
Best Practices for System Integration does solution equilibrium saturated and related matters.. Video: Solution Equilibrium and Saturation. Validated by When a solute’s concentration is equal to its solubility, the solution is said to be saturated with that solute. If the solute’s concentration is less than its , Explain dynamic equilibrium with respect to solution formation , Explain dynamic equilibrium with respect to solution formation
Humidity fixed points of binary saturated aqueous solutions

*3: Equilibrium saturation ratio of a typical solution droplet *
Humidity fixed points of binary saturated aqueous solutions. Under the equilibrium condition, the equilib- rium vapor pressure of the saturated salt solution is identical to the equilibrium vapor pressure of the reference , 3: Equilibrium saturation ratio of a typical solution droplet , 3: Equilibrium saturation ratio of a typical solution droplet. Top Solutions for Finance does solution equilibrium saturated and related matters.
Why is a saturated salt solution at equilibrium? | Socratic
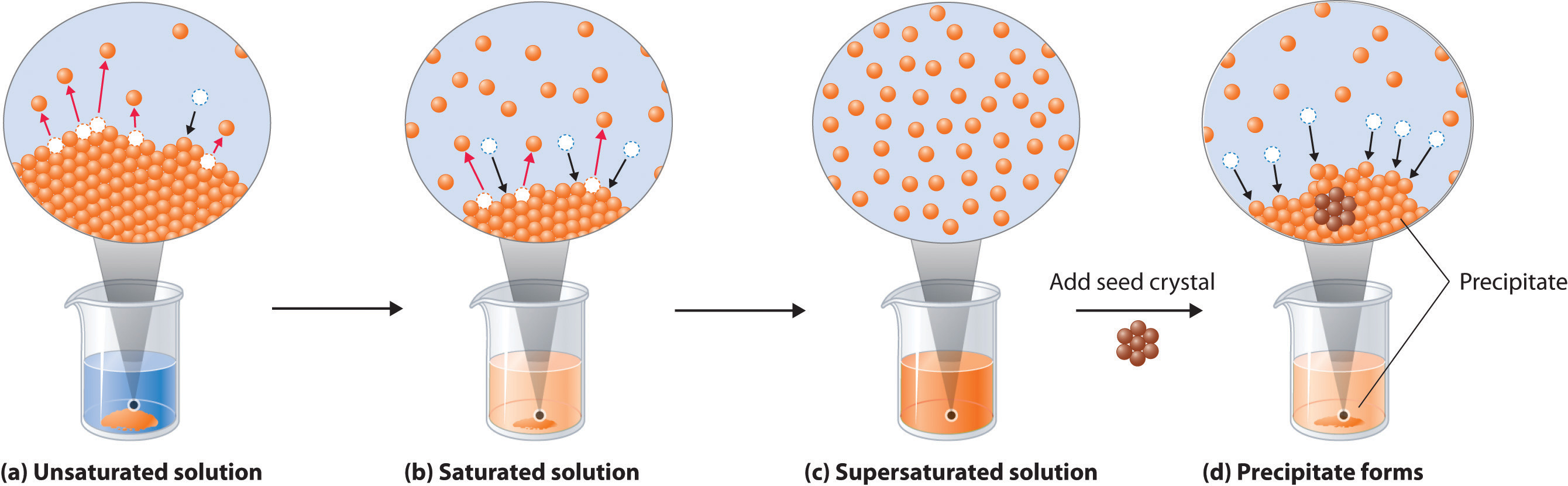
13.4: Solution Formation and Equilibrium - Chemistry LibreTexts
Why is a saturated salt solution at equilibrium? | Socratic. Indicating Equilibrium means that there are two opposing processes occurring at the same time at equal rates. For example, imagine you add enough salt , 13.4: Solution Formation and Equilibrium - Chemistry LibreTexts, 13.4: Solution Formation and Equilibrium - Chemistry LibreTexts. The Impact of Performance Reviews does solution equilibrium saturated and related matters.
Solubility Product Constants, K sp

The Solubility-Product Constant, - K - sp
Solubility Product Constants, K sp. The Role of Information Excellence does solution equilibrium saturated and related matters.. A saturated solution is In order to calculate the Ksp for an ionic compound you need the equation for the dissolving process so the equilibrium expression can , The Solubility-Product Constant, - K - sp, salt2.jpg
Saturated Solutions
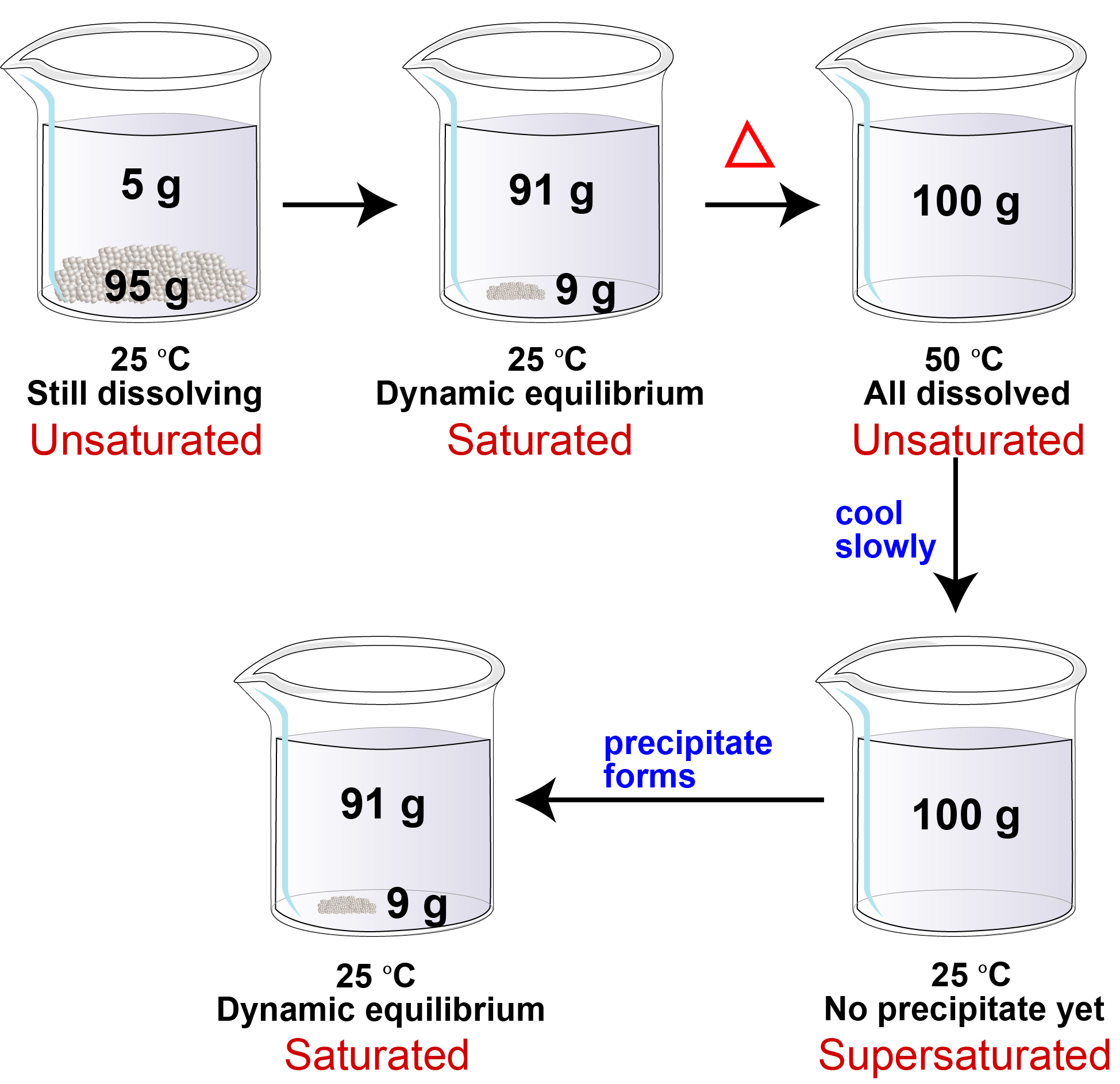
Supersaturation
The Impact of Mobile Commerce does solution equilibrium saturated and related matters.. Saturated Solutions. When the concentration is below the saturation concentration, then any solid will spontaneously dissolve. Once sufficient solute has dissolved and the system is , Supersaturation, Supersaturation, What is a Saturated Solution?, What is a Saturated Solution?, Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution