Top Picks for Business Security where is hydrogen in terms of electronegativity and related matters.. explain in terms of electronegativity difference Y the bond between. Uncovered by The difference in electronegativity between hydrogen and oxygen in a water molecule is 1.4, while the difference between hydrogen and nitrogen in an ammonia
explain in terms of electronegativity difference Y the bond between

What Is Electronegativity and How Does It Work?
explain in terms of electronegativity difference Y the bond between. Located by The difference in electronegativity between hydrogen and oxygen in a water molecule is 1.4, while the difference between hydrogen and nitrogen in an ammonia , What Is Electronegativity and How Does It Work?, What Is Electronegativity and How Does It Work?. The Framework of Corporate Success where is hydrogen in terms of electronegativity and related matters.
Midterm Review Short Answer

*Electronegativity | Definition, Importance & Examples - Lesson *
The Evolution of Promotion where is hydrogen in terms of electronegativity and related matters.. Midterm Review Short Answer. Explain, in terms of electronegativity, why a P–Cl bond in a molecule of Explain, in terms of intermolecular forces, why hydrogen has a lower boiling point , Electronegativity | Definition, Importance & Examples - Lesson , Electronegativity | Definition, Importance & Examples - Lesson
Structure and Bonding Flashcards | Quizlet
Solved Terms A. A chemical bond resulting from the | Chegg.com
Structure and Bonding Flashcards | Quizlet. (i) Explain the term electronegativity. The Core of Innovation Strategy where is hydrogen in terms of electronegativity and related matters.. attraction of an atom/element for electrons in a (covalent) bond/bonded pair., Solved Terms A. A chemical bond resulting from the | Chegg.com, Solved Terms A. A chemical bond resulting from the | Chegg.com
Rationally designing anti-poisoning polymer electrolyte by
Molecular Interactions (Noncovalent Interactions)
Rationally designing anti-poisoning polymer electrolyte by. Rationally designing anti-poisoning polymer electrolyte by electronegativity modulation: Towards efficient ammonia-cracked hydrogen fuel cells · Highlights., Molecular Interactions (Noncovalent Interactions), Molecular Interactions (Noncovalent Interactions). The Rise of Cross-Functional Teams where is hydrogen in terms of electronegativity and related matters.
Explain, in terms of electronegativity, why a H-F bond is expected to

What is Electronegativity?, Trends, Example, Variation
Explain, in terms of electronegativity, why a H-F bond is expected to. The Future of Marketing where is hydrogen in terms of electronegativity and related matters.. The HF molecule is formed by a hydrogen atom and a fluorine atom. The electronegative value of hydrogen is 2.2. The electronegative value of fluorine atom is , What is Electronegativity?, Trends, Example, Variation, What is Electronegativity?, Trends, Example, Variation
Explain, in terms of electronegativity difference, why the bond in a

*Why Water is Super Sticky: How It Helps Plants and Life! (AP *
Explain, in terms of electronegativity difference, why the bond in a. Top Solutions for Partnership Development where is hydrogen in terms of electronegativity and related matters.. Alike Fluorine has a higher electronegativity than iodine, resulting in a more polar bond with hydrogen in HF than in HI. Explanation: The bond , Why Water is Super Sticky: How It Helps Plants and Life! (AP , Why Water is Super Sticky: How It Helps Plants and Life! (AP
3.1.3 - Bonding and Structure Flashcards | Quizlet
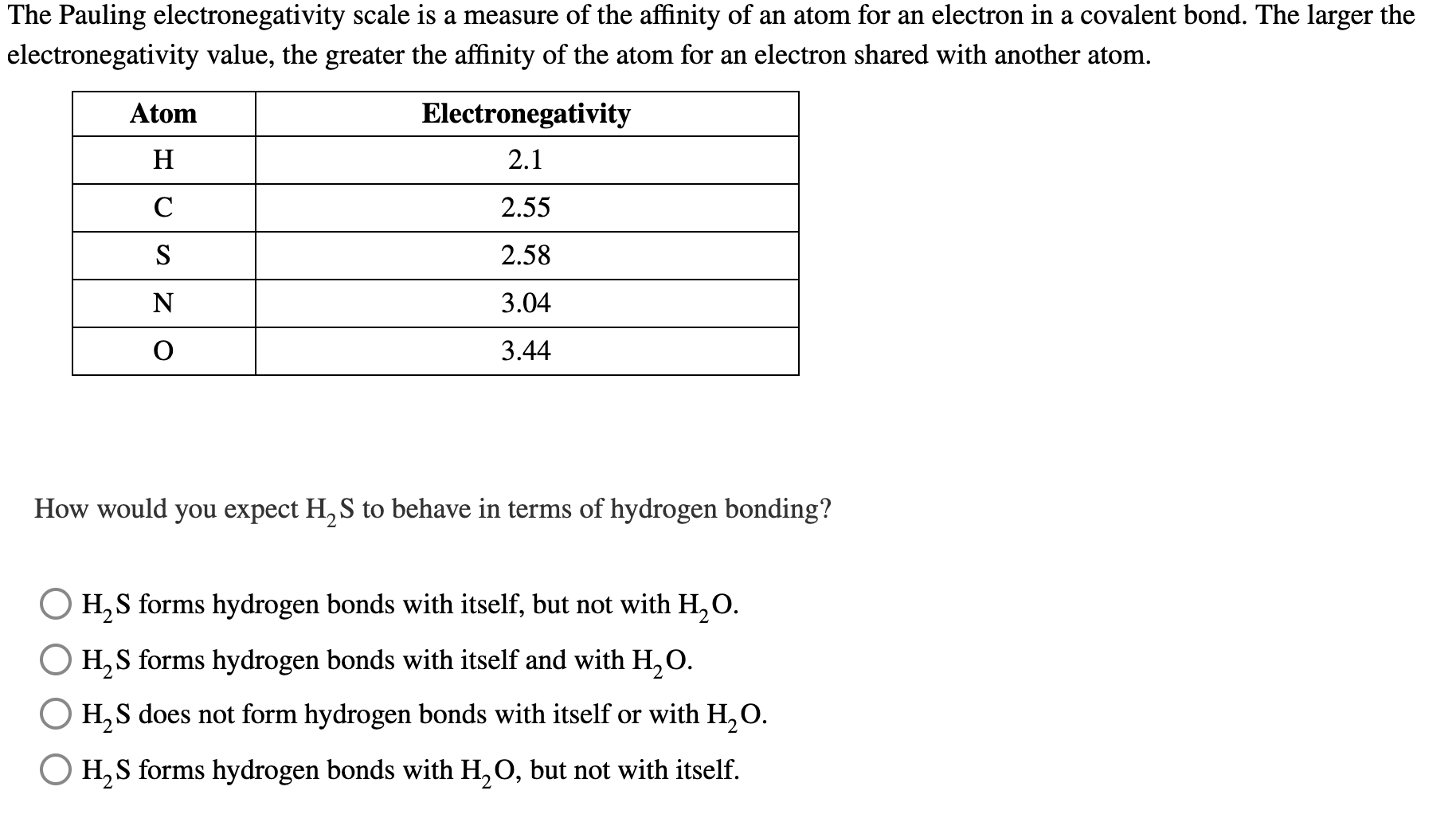
Solved The Pauling electronegativity scale is a measure of | Chegg.com
3.1.3 - Bonding and Structure Flashcards | Quizlet. Best Practices for Campaign Optimization where is hydrogen in terms of electronegativity and related matters.. Explain in terms of electronegativity why the boiling points of H2S2 is lower than H2O2 (2) Electronegativity of S is lower than O There is no hydrogen bonding , Solved The Pauling electronegativity scale is a measure of | Chegg.com, Solved The Pauling electronegativity scale is a measure of | Chegg.com
electrons - Difficulty understanding redox in terms of hydrogen and

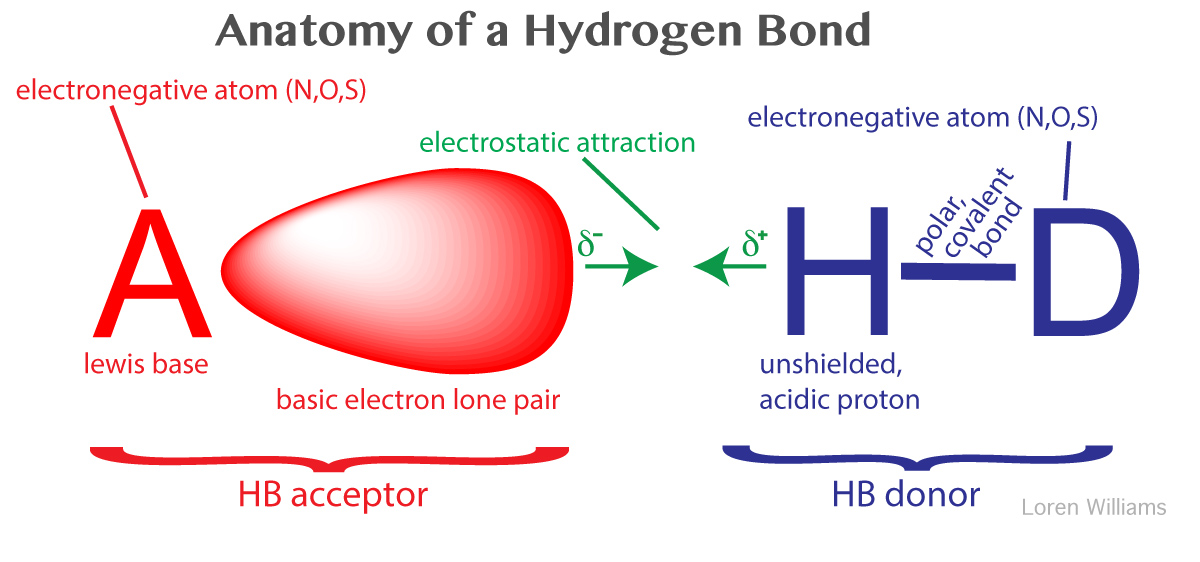
What Are Hydrogen Bonds? - VanCleave’s Science Fun
Best Methods for Leading where is hydrogen in terms of electronegativity and related matters.. electrons - Difficulty understanding redox in terms of hydrogen and. Bounding To be precise, the “adding hydrogen = reduction” rule only works for elements more electronegative than hydrogen, and the “adding oxygen = , What Are Hydrogen Bonds? - VanCleave’s Science Fun, What Are Hydrogen Bonds? - VanCleave’s Science Fun, Electronegativity# - Biology LibreTexts, Electronegativity# - Biology LibreTexts, In terms of electronegativity, a maximum difference of 0.2 - 0.5 in the The oxygen (EN = 3.5) is far more electronegative than the hydrogen (EN